Introduction of Quality System
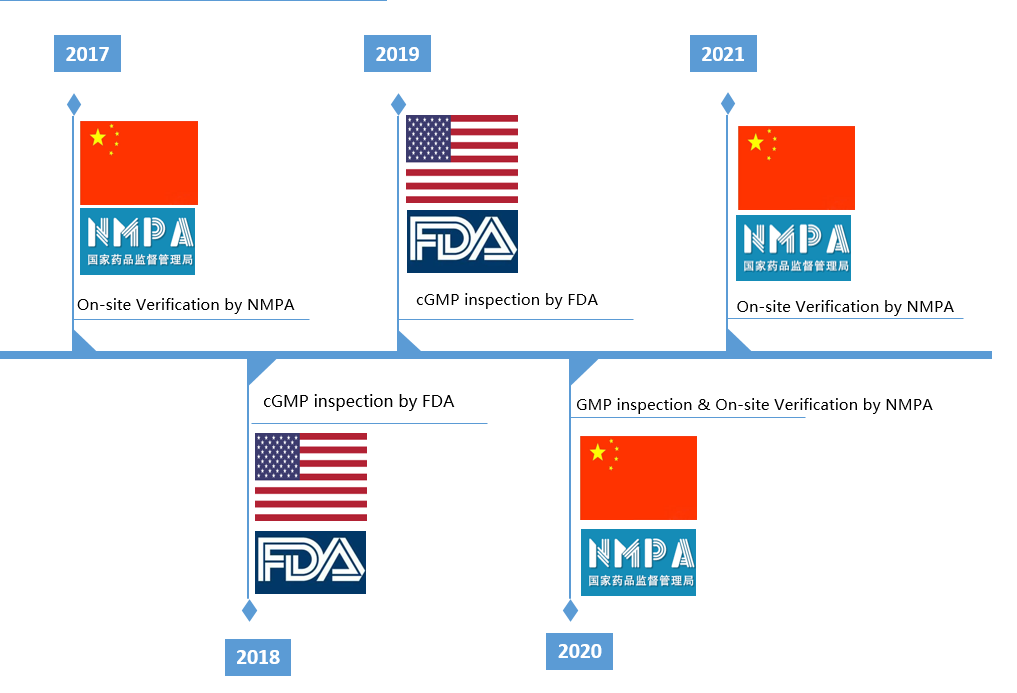
Adhering to the concept of "Safety, Efficiency and Controllability", and on the basis of the regulations stipulated by Part 210 & 211, US21 CFR, cGMP, ICH and other domestic and international regulations, Bostal has established a set of quality management system covering the entire process of drug R&D and commercial manufacturing, which complies with standard regulations of both China and USA. Bostal follows the quality policy of life first, quality first, and benefiting to human health.
The current Good Manufacturing Practice (cGMP) is a set of standards that focus on quality management system. Bostal pursues excellence in quality management and has established a quality management system that conforms to the cGMP and other relevant regulations in China and USA, and continues to standardize and improve our various production and quality management procedures according to the updates of the regulations. We have established a quality control center with complete and advanced testing instruments and electronic data processing system that meets the requirements of data reliability. This system is supported by a quality team that has been well trained and assessed to meet revelant requirements, they organize the testing and release of materials, intermediate products and finished products in a standardized and orderly manner to ensure the accuracy and reliability of testing data to guarantee the quality of products.
In order to improve the quality management system of drug R&D and commercial manufacturing, and to simplify bussiness opreation process, we have introduced ERP, QMS, LIMS, PMS and PQS systems. By using the information technologies, we can sort out various business processes of drug products manufacturing, quality control, quality assurance, warehousing supply, equipment management and project management, and improve the efficiency of enterprises on the basis of regulatory compliance.
We always implement the practice of cGMP concept to provide continuous professional services to our partners with the highest standards of quality in the pharmaceutical industry globally, and provide quality medicines to patients worldly.